General considerations for participants requiring an invasive procedure or surgery:
Consider whether patient-related factors that impart increased bleed risk are present. For example, uncontrolled hypertension, altered coagulation profile, abnormal liver function, age ≥75 years, anaemia, thrombocytopenia, and concomitant use of aspirin.
2. Assess the bleeding risk associated with the procedure:
i. no clinically important bleed risk;
ii. low procedural bleed risk;
iii. uncertain procedural bleed risk; or
iv. intermediate/high procedural bleed risk.
*A list of common procedures and associated procedural bleed risk can be found in this document. The proceduralist’s opinion of bleed risk may vary from that proposed in this document.
3. Consider the clinical effect of bleeding should it occur;
4. Whether the participant requires neuraxial anaesthesia, and
5. If the invasive procedure is an elective or emergency procedure.
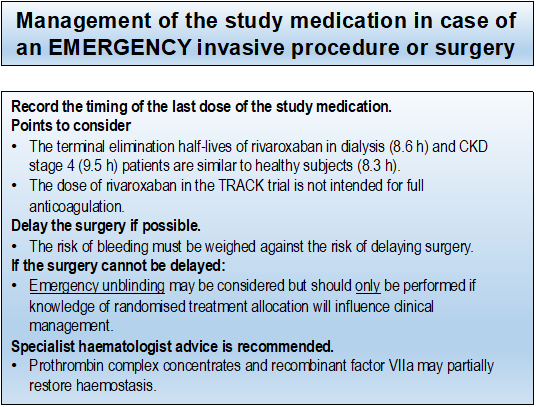
If surgery is urgent or needed in an emergency situation, the risk of bleeding must be weighed against the risk of delaying surgery. In people with kidney failure receiving haemodialysis, a trial of rivaroxaban 10 mg daily resulted in exposure similar to that of 20 mg in healthy volunteers. The total daily dose in participants randomised to receive rivaroxaban 2.5 mg twice daily is half of that required for full anticoagulation in people with kidney failure receiving haemodialysis. However, there are no reliable data or evidence-based guidelines in people taking any NOAC and requiring urgent surgery with normal or decreased kidney function.
If the invasive procedure/surgery cannot be delayed, specialist haematology advice is strongly recommended for consideration of the use of prothrombin complex concentrates (PCC), activated prothrombin complex concentrates (APCC) or recombinant factor VIIa, or reversal with andexanet alfa where available (it is currently not approved for perioperative management). Consider platelet transfusion in case of thrombocytopenia and/or if the participant is on aspirin.
Note that normal values of prothrombin time (PT), INR and activated partial thromboplastin time (aPTT) do not exclude anticoagulant effect of rivaroxaban. If it is possible to obtained rivaroxaban-calibrated anti-Xa level in a timely fashion, clinicians may use this information to decide on the appropriateness of not delaying the invasive procedure/surgery.
If there is a concern about the risk of bleeding in a participant who requires urgent or emergency surgery, unblinding may be considered but should only be performed if knowledge of randomised treatment allocation will influence clinical management.