|
|
Study Overview
The TRACK trial will evaluate whether a small dose of
rivaroxaban, a blood-thinning medication, would reduce cardiovascular
death or major cardiovascular events in patients with advanced stages
of chronic kidney disease.
|
|
|
|
Important Updates & Reminders
|
|
|
1. Participant
Status
Retention of participants is key to the success of the
TRACK trial. Every participant counts. For the trial to succeed, we
need to do everything we can to keep the participants in the trial even
when they discontinued the study medication.
Run-In
The run-in period is 21 days (range 14 to 30
days). Once the participant is enrolled into the trial, it is
important to randomise the participant within
this required timeframe. Tip: As part
of the screening visit, book the participant randomisation
visit for as soon as possible after the end of the 21 days period,
so it will allow time to follow up the participant if needed within the
30 days window.
Withdrawn- Pre-Randomisation
All the trial procedures should be discussed with
participants in the screening visit. This may help to reduce the
number of participants who decide to not continue in the trial
after the run-in period.
Withdrawn-Post-Randomisation
Once the participant has been randomised to the trial,
there is no need to withdraw a participant. Usually when a
participant asks to withdraw from the trial, they want to stop
taking the study medication. In this case, the site PI
should discuss with the participant their options.
Discussion points & participant options:
·
Ask the participant
why they want to withdraw from the trial.
·
If the participant wants
to stop taking the study medication, explain that they do not have to
withdraw from the trial to stop taking the study medication. Ask
the participant to continue with the study follow ups and let them know
that all follow up visits can be also conducted remotely, via
video calls, phone or telehealth.
·
If the participant
decides that they no longer want to be contacted for the study, ask the
participant for permission to continue collecting information that
does not require participant contact. It will allow us to continue
collecting study events that can be ascertained from the medical
records, or by contacting other health practitioners.
|
|
|
2.
Database: Mid-Study Update #3
The TRACK database Mid-Study Update #3 is now completed.
The following changes have been made:
·
Exclusion
Criteria: Additional exclusion criteria:
‘Incapable of recognizing the nature, significance, and scope of the
clinical trial or giving consent are excluded, even if they have a
legal representative’. Note: This exclusion criteria needs
to be completed by German sites only, all sites outside Germany
need to tick 'Not applicable' to this
criteria.
·
Enrolment – Visit
Date: Hiding or inability for site staff to answer the
question ‘MBS and/or PBS consent obtained?’
·
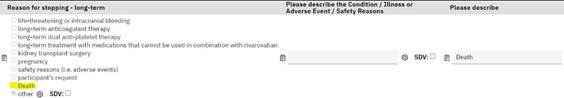
Study Medication
Log: Addition of ‘Death’ option if ‘Long-term’ IMP stoppage
is selected.
Previously if the reason for stopping IMP long term was
death, then sites ticked 'Other' and added 'Death' under
'Please describe'. To assist in analysing the data at later
stage, we would like sites to change their response from
'Other' to 'Death'. Please see below a screenshot from the
database of the change required:
|
|
|
|
|
Input caption text here. Use the block's Settings tab to
change the caption position and set other styles.
|
|
|
Presentation by Professor Sunil Badve
|
|
|

|
|
Professor Sunil Badve presentation regarding the TRACK
trial at the Nephrology Grand Rounds at the Ottawa Hospital, Canada
|
|
|
Recruitment and Country Updates
All figures are as of 24 April 2023
|
|
|
Global Recruitment Graph: Monthly Progression
Below figures are as of 24 April 2023
|
|
|
Global Recruitment Graph: Randomised vs Target
Below figures are as of 24 April 2023
|
|
|
We would like to warmly welcome the new site Research
St. Joseph's - Hamilton that is now activated for the TRACK
trial:
Country: Canada
Site Name: Research St. Joseph's - Hamilton
Site PI: Dr. Michael Walsh
Date Site Activation: 24 February 2023
We are looking forward to seeing the first participant enrolled in your
site!
|
|
|
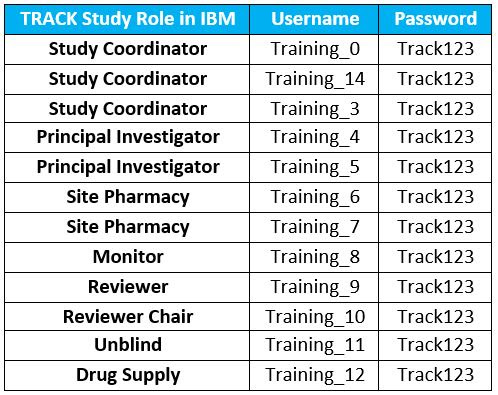
IBM
Training Database Logins
For any site staff who wish to train/practice
data entry for the study, please visit the TRACK Training IBM Database
by logging in with any of the following updated training accounts.
|
|
|
|
|

|
|
Name: Prof Jannet Labidi
Position/Role: TRACK Tunisian Principal Investigator
Location: Tunis, Tunisia
About: Professor Labidi is the Head
of the Nephrology Department at Military Hospital, Tunis, Tunisia. She
obtained Nephrology diploma since 2000, and additional diploma in
methodology, statistics and epidemiology as well as European Diploma in
First Aid and Basic Cardiopulmonary Resuscitation.
She is passionate about the research in her field and is member of the
research unit in the hemodialysis department,
member of research laboratory LR12DN01 entitled "Hemodynamic
Resuscitation and Techniques Extrarenal Purification", member of
the commission responsible for developing medical standards of
suitability for recruitment for the benefit of the army, with the
production of a regulatory test and member of a working group whose objective
is the development of a Tunisian reference system. She is also the
president of the TUN-CKDD project evaluating the prevalence of chronic
kidney disease in Tunisian diabetics.
|
|
|
|