|
|
|
🎇Special
Message from the TRACK Chief Investigators🎇 Dear TRACK site
teams, As we approach
the end of 2024, we want to express our deepest gratitude for your invaluable
contributions to the trial. We are now transitioning toward closing
recruitment in most countries. Thanks to your dedication and efforts, we have
reached the
significant milestone of enrolling 1,300 participants. A
special thanks to our colleagues in Germany, Belgium, France, Nepal and some
sites in Australia and India, who will continue enrolling participants until
June 30, 2025. As we move to
the next phase of the trial, it is important that we focus to focus on
three main points: 1.
Participant
retention: Maintaining participant retention is vital to
preserving the scientific integrity of the trial. Please strive to minimize
withdrawals, as high withdrawal rates can undermine the study's validity.
When in-person follow-ups are not feasible, consider offering alternative
follow-up options. 2.
Minimise treatment
discontinuation: High discontinuation rates of the study
medication hinder our ability to accurately evaluate the effectiveness and
risk-benefit profile of low-dose rivaroxaban. Whenever possible, minimize
extended treatment interruptions. If medically appropriate, consider resuming
the study medication—even after an extended pause 3.
Reporting trial
outcomes: Every participant and every outcome matter. As an
event-driven trial, TRACK depends on timely reporting of outcomes by sites.
Regularly reviewing medical records is essential to ensure no outcomes are
overlooked. If you have any uncertainties, please do not hesitate to reach
out to us. Your
dedication to TRACK is essential to our mission. By working together, we can
ensure the TRACK trial is completed successfully and on time, making a
lasting impact on the lives of patients with kidney disease. Merry
Christmas and Happy New Year Sunil and
Martin |
|
Overview of Recruitment and
Outcomes |
|
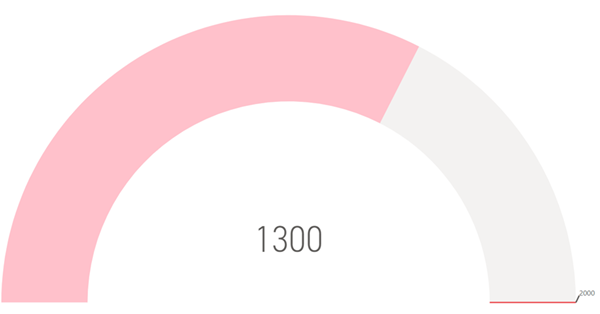
🎉 Recruitment
Target 🎉 A huge thank you again to everyone for contributing
to our goal of 1300 by December 31st 2024,
our goal was achieved with time to spare!!! |
|
|
|
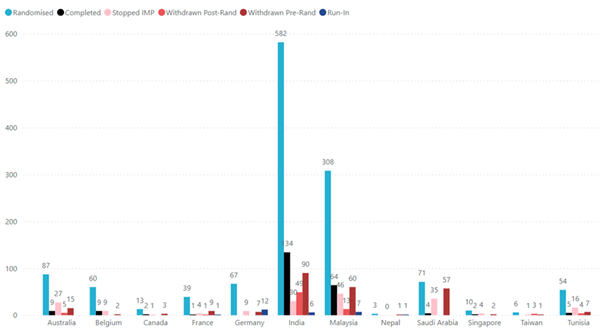
Recruitment by Location
Total Participants
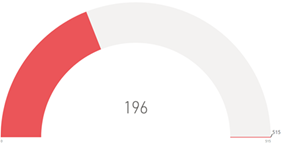
Experiencing 1st Primary Event
|
|
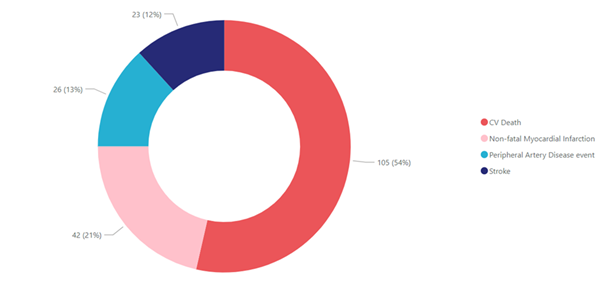
1st Primary Event |
|
*All figures
are as of 17 Dec 2024 |
|
📢 Important
Reminders: 📢 ·
When completing the Concomitant Medication page in the EDC, please
first check what medications the participant is currently taking and then
select from the pre-defined categories that match the medications being
taken. “Other” is only for medications NOT covered by the categories
listed on the Concomitant Page. ·
Please remember to update the Study Medication Log in the EDC for any
short or long terms IMP stoppages. Be careful to select the correct
category for the reason for the stoppage and check if it is a medical event
whether it should also be reported as an outcome or SAE ·
When completing the SAE form, the information provided in “Main
Diagnosis” should only include medical terminology and be the main diagnosis
for the admission. Please only use medical terminology as the terms
will be mapped using a medical dictionary and this cannot be done if too much
information is provided. Additional information should be included in
the section “Briefly describe the event” ·
Please remember that “thrombosis of dialysis vascular access among
participants with an AV fistula/graft” does NOT include “dialysis catheter
thrombosis” |
|
End of
Recruitment – Summary 1.
ALL sites in Canada, Singapore, Taiwan, Saudi Arabia, Tunisia and Malaysia
will close to enrolment in December 2024. 2.
The majority of sites in India and Australia will close in December 2024 with
a few remaining open until June 2025. 3.
ALL sites in Belgium, Germany, France and Nepal will remain open until June
2025. |
|
IBM Training
Database Logins For any site staff
who wish to train/practice data entry for the study, please visit the TRACK
Training IBM Database by logging in with any of the following updated
training accounts. |
|
Thank you for supporting
the Track Trial. To learn more please visit www.tracktrial.org Warm regards, The TRACK
Trial team
|